Help us make food transparency the norm!
As a non-profit organization, we depend on your donations to continue informing consumers around the world about what they eat.
The food revolution starts with you!
Crackers - Ferrari Ghezzi - 200 g
Crackers - Ferrari Ghezzi - 200 g
Barra-kodea: 7771223000018 (EAN / EAN-13)
Izen arrunta: galletas de harina de trigo fortificada.
Kopurua: 200 g
Ontziratzea: en:Plastic, PP, en:Bag
Markak: Ferrari Ghezzi
Kategoriak: en:Snacks, en:Salty snacks, en:Sweet snacks, en:Appetizers, en:Biscuits and cakes, Gaileta, en:Crackers
Etiketak, ziurtagiriak, sariak: en:No cholesterol
Origin of ingredients: Bolivia
Manufacturing or processing places: Oruro, Bolivia
Traceability code: 050103060001
Link to the product page on the official site of the producer: https://sfida.com.bo/producto/crackers-2...
Dendak: Pasteur
Matching with your preferences
Health
Osagaiak
-
16 ingredients
: Harina de trigo fortificada (Hierro, Vitaminas (B1, B2), niacina, ácido fólico), Azúcar, Manteca vegetal (Soya), Emulsionante (E322), Estabilizante (Extracto de malta), Leudante químico (E500ii), sal yodada.Alergenoak: en:Gluten, en:Soybeans
Food processing
-
Ultra processed foods
Elements that indicate the product is in the 4 - Ultra prozesatutako elikagaiak eta edariak group:
- Gehigarria: E322
- Osagaia: Emulsifier
Food products are classified into 4 groups according to their degree of processing:
- Prozesatu gabeko edo ahalik eta gutxien prozesatutako elikagaiak
- Sukaldaritzako osagaiak prozesatu
- Prozesatutako jakiak
- Ultra processed foods
The determination of the group is based on the category of the product and on the ingredients it contains.
Gehigarriak
-
E322
Lecithin: Lecithin -UK: , US: , from the Greek lekithos, "egg yolk"- is a generic term to designate any group of yellow-brownish fatty substances occurring in animal and plant tissues, which are amphiphilic – they attract both water and fatty substances -and so are both hydrophilic and lipophilic-, and are used for smoothing food textures, dissolving powders -emulsifying-, homogenizing liquid mixtures, and repelling sticking materials.Lecithins are mixtures of glycerophospholipids including phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine, and phosphatidic acid.Lecithin was first isolated in 1845 by the French chemist and pharmacist Theodore Gobley. In 1850, he named the phosphatidylcholine lécithine. Gobley originally isolated lecithin from egg yolk—λέκιθος lekithos is "egg yolk" in Ancient Greek—and established the complete chemical formula of phosphatidylcholine in 1874; in between, he had demonstrated the presence of lecithin in a variety of biological matters, including venous blood, in human lungs, bile, human brain tissue, fish eggs, fish roe, and chicken and sheep brain. Lecithin can easily be extracted chemically using solvents such as hexane, ethanol, acetone, petroleum ether, benzene, etc., or extraction can be done mechanically. It is usually available from sources such as soybeans, eggs, milk, marine sources, rapeseed, cottonseed, and sunflower. It has low solubility in water, but is an excellent emulsifier. In aqueous solution, its phospholipids can form either liposomes, bilayer sheets, micelles, or lamellar structures, depending on hydration and temperature. This results in a type of surfactant that usually is classified as amphipathic. Lecithin is sold as a food additive and dietary supplement. In cooking, it is sometimes used as an emulsifier and to prevent sticking, for example in nonstick cooking spray.Source: Wikipedia (Ingeles)
-
E500
Sodium carbonate: Sodium carbonate, Na2CO3, -also known as washing soda, soda ash and soda crystals, and in the monohydrate form as crystal carbonate- is the water-soluble sodium salt of carbonic acid. It most commonly occurs as a crystalline decahydrate, which readily effloresces to form a white powder, the monohydrate. Pure sodium carbonate is a white, odorless powder that is hygroscopic -absorbs moisture from the air-. It has a strongly alkaline taste, and forms a moderately basic solution in water. Sodium carbonate is well known domestically for its everyday use as a water softener. Historically it was extracted from the ashes of plants growing in sodium-rich soils, such as vegetation from the Middle East, kelp from Scotland and seaweed from Spain. Because the ashes of these sodium-rich plants were noticeably different from ashes of timber -used to create potash-, they became known as "soda ash". It is synthetically produced in large quantities from salt -sodium chloride- and limestone by a method known as the Solvay process. The manufacture of glass is one of the most important uses of sodium carbonate. Sodium carbonate acts as a flux for silica, lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass produced insoluble. This type of glass is known as soda lime glass: "soda" for the sodium carbonate and "lime" for the calcium carbonate. Soda lime glass has been the most common form of glass for centuries. Sodium carbonate is also used as a relatively strong base in various settings. For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It acts as an alkali because when dissolved in water, it dissociates into the weak acid: carbonic acid and the strong alkali: sodium hydroxide. This gives sodium carbonate in solution the ability to attack metals such as aluminium with the release of hydrogen gas.It is a common additive in swimming pools used to raise the pH which can be lowered by chlorine tablets and other additives which contain acids. In cooking, it is sometimes used in place of sodium hydroxide for lyeing, especially with German pretzels and lye rolls. These dishes are treated with a solution of an alkaline substance to change the pH of the surface of the food and improve browning. In taxidermy, sodium carbonate added to boiling water will remove flesh from the bones of animal carcasses for trophy mounting or educational display. In chemistry, it is often used as an electrolyte. Electrolytes are usually salt-based, and sodium carbonate acts as a very good conductor in the process of electrolysis. In addition, unlike chloride ions, which form chlorine gas, carbonate ions are not corrosive to the anodes. It is also used as a primary standard for acid-base titrations because it is solid and air-stable, making it easy to weigh accurately.Source: Wikipedia (Ingeles)
-
E500ii - Sodio hidrogenokarbonato
Sodium carbonate: Sodium carbonate, Na2CO3, -also known as washing soda, soda ash and soda crystals, and in the monohydrate form as crystal carbonate- is the water-soluble sodium salt of carbonic acid. It most commonly occurs as a crystalline decahydrate, which readily effloresces to form a white powder, the monohydrate. Pure sodium carbonate is a white, odorless powder that is hygroscopic -absorbs moisture from the air-. It has a strongly alkaline taste, and forms a moderately basic solution in water. Sodium carbonate is well known domestically for its everyday use as a water softener. Historically it was extracted from the ashes of plants growing in sodium-rich soils, such as vegetation from the Middle East, kelp from Scotland and seaweed from Spain. Because the ashes of these sodium-rich plants were noticeably different from ashes of timber -used to create potash-, they became known as "soda ash". It is synthetically produced in large quantities from salt -sodium chloride- and limestone by a method known as the Solvay process. The manufacture of glass is one of the most important uses of sodium carbonate. Sodium carbonate acts as a flux for silica, lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass produced insoluble. This type of glass is known as soda lime glass: "soda" for the sodium carbonate and "lime" for the calcium carbonate. Soda lime glass has been the most common form of glass for centuries. Sodium carbonate is also used as a relatively strong base in various settings. For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It acts as an alkali because when dissolved in water, it dissociates into the weak acid: carbonic acid and the strong alkali: sodium hydroxide. This gives sodium carbonate in solution the ability to attack metals such as aluminium with the release of hydrogen gas.It is a common additive in swimming pools used to raise the pH which can be lowered by chlorine tablets and other additives which contain acids. In cooking, it is sometimes used in place of sodium hydroxide for lyeing, especially with German pretzels and lye rolls. These dishes are treated with a solution of an alkaline substance to change the pH of the surface of the food and improve browning. In taxidermy, sodium carbonate added to boiling water will remove flesh from the bones of animal carcasses for trophy mounting or educational display. In chemistry, it is often used as an electrolyte. Electrolytes are usually salt-based, and sodium carbonate acts as a very good conductor in the process of electrolysis. In addition, unlike chloride ions, which form chlorine gas, carbonate ions are not corrosive to the anodes. It is also used as a primary standard for acid-base titrations because it is solid and air-stable, making it easy to weigh accurately.Source: Wikipedia (Ingeles)
Ingredients analysis
-
en:May contain palm oil
Ingredients that may contain palm oil: en:Vegetable fat
-
en:Vegan status unknown
Unrecognized ingredients: Burdina, Tiamina, Azido foliko
-
en:Vegetarian status unknown
Unrecognized ingredients: Burdina, Tiamina, Azido foliko
-
Details of the analysis of the ingredients
: Harina de trigo fortificada (Hierro, vitaminas, vitamina B1, vitamina B2, niacina, ácido fólico), Azúcar, Manteca vegetal, Emulsionante (e322), Estabilizante (Extracto de malta), Leudante químico (e500ii), sal yodada- Harina de trigo fortificada -> en:fortified-wheat-flour - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 9410 - percent_min: 14.2857142857143 - percent_max: 100
- Hierro -> en:iron - percent_min: 2.38095238095238 - percent_max: 100
- vitaminas -> en:vitamins - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 50
- vitamina B1 -> en:thiamin - percent_min: 0 - percent_max: 33.3333333333333
- vitamina B2 -> en:e101 - vegan: maybe - vegetarian: yes - percent_min: 0 - percent_max: 25
- niacina -> en:e375 - vegan: maybe - vegetarian: maybe - percent_min: 0 - percent_max: 20
- ácido fólico -> en:folic-acid - percent_min: 0 - percent_max: 16.6666666666667
- Azúcar -> en:sugar - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016 - percent_min: 0 - percent_max: 50
- Manteca vegetal -> en:vegetable-fat - vegan: yes - vegetarian: yes - from_palm_oil: maybe - percent_min: 0 - percent_max: 33.3333333333333
- Emulsionante -> en:emulsifier - percent_min: 0 - percent_max: 25
- e322 -> en:e322 - vegan: maybe - vegetarian: maybe - percent_min: 0 - percent_max: 25
- Estabilizante -> en:stabiliser - percent_min: 0 - percent_max: 20
- Extracto de malta -> en:malt-extract - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 20
- Leudante químico -> en:raising-agent - percent_min: 0 - percent_max: 16.6666666666667
- e500ii -> en:e500ii - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 16.6666666666667
- sal yodada -> en:iodised-salt - vegan: yes - vegetarian: yes - ciqual_food_code: 11058 - percent_min: 0 - percent_max: 14.2857142857143
- Harina de trigo fortificada -> en:fortified-wheat-flour - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 9410 - percent_min: 14.2857142857143 - percent_max: 100
Elikadura
-
Missing data to compute the Nutri-Score
Missing nutrition facts
⚠ ️The nutrition facts of the product must be specified in order to compute the Nutri-Score.Could you add the information needed to compute the Nutri-Score? Add nutrition facts
-
Nutrient levels
-
Koipe in moderate quantity (10.2%)
What you need to know- A high consumption of fat, especially saturated fats, can raise cholesterol, which increases the risk of heart diseases.
Recommendation: Limit the consumption of fat and saturated fat- Choose products with lower fat and saturated fat content.
-
Gantz-azido ase in low quantity (0.2%)
What you need to know- A high consumption of fat, especially saturated fats, can raise cholesterol, which increases the risk of heart diseases.
Recommendation: Limit the consumption of fat and saturated fat- Choose products with lower fat and saturated fat content.
-
-
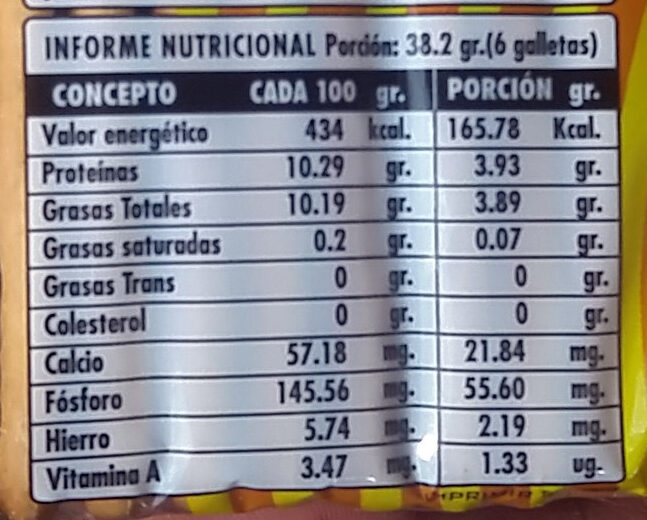
Nutrition facts
Nutrition facts As sold
for 100 g / 100 mlAs sold
per serving (6 galletas 38.2 g)Compared to: en:Crackers Energia 1.816 kj
(434 kcal)694 kj
(166 kcal)-% 4 Koipe 10,19 g 3,89 g -% 39 Gantz-azido ase 0,2 g 0,076 g -% 94 Trans fat 0 g 0 g Kolesterol 0 mg 0 mg -% 100 Carbohydrates - - Azukre - - Fiber - - Proteina 10,29 g 3,93 g +% 11 Gatz arrunt - - A bitamina 3,47 µg 1,33 µg +% 799 Kaltzio 57,18 mg 21,8 mg +% 21 Fosforo 145,56 mg 55,6 mg Burdina 5,74 mg 2,19 mg +% 28 Fruits‚ vegetables‚ nuts and rapeseed‚ walnut and olive oils (estimate from ingredients list analysis) 0 % 0 %
Ingurumena
-
Eco-Score B - Ingurumen-eragin txikia
The Eco-Score is an experimental score that summarizes the environmental impacts of food products.→ The Eco-Score was initially developped for France and it is being extended to other European countries. The Eco-Score formula is subject to change as it is regularly improved to make it more precise and better suited to each country.Life cycle analysis
-
Average impact of products of the same category: A (Score: 87/100)
Kategoria: Salty snacks, crackers, plain
Kategoria: Salty snacks, crackers, plain
- PEF environmental score: 0.22 (the lower the score, the lower the impact)
- including impact on climate change: 1.59 kg CO2 eq/kg of product
Stage Impact Agriculture
59.7 %Processing
22.4 %Ontziratzea
10.6 %Transportation
5.2 %Distribution
2.2 %Consumption
0.0 %
Bonuses and maluses
-
Origins of ingredients with a high impact
Malus: -5
Environmental policy: -5
Transportation: 0
Origin of the product and/or its ingredients % of ingredients Impact Bolivia 100 %Altua
-
Packaging with a medium impact
Malus: -8
Shape Material Recycling Impact 1 Bag PP Recycle Altua
Eco-Score for this product
-
Impact for this product: B (Score: 74/100)
Produktua: Crackers - Ferrari Ghezzi - 200 g
Life cycle analysis score: 87
Sum of bonuses and maluses: -13
Final score: 74/100
-
Carbon footprint
-
Equal to driving 0.8 km in a petrol car
159 g CO² per 100g of product
The carbon emission figure comes from ADEME's Agribalyse database, for the category: Salty snacks, crackers, plain (Source: ADEME Agribalyse Database)
Stage Impact Agriculture
53.6 %Processing
18.8 %Ontziratzea
17.7 %Transportation
8.7 %Distribution
1.2 %Consumption
0.0 %
Ontziratzea
-
Packaging with a medium impact
-
Packaging parts
1 x Bag (PP)
-
Bilgarriaren materialak
Material % Bilgarriaren pisua Bilgarriaren pisua produktuaren 100g-ko Plastic
-
Transportation
-
Origins of ingredients
Origins of ingredients with a high impact
Origin of the product and/or its ingredients % of ingredients Impact Bolivia 100 %Altua
Report a problem
-
Incomplete or incorrect information?
Category, labels, ingredients, allergens, nutritional information, photos etc.
If the information does not match the information on the packaging, please complete or correct it. Open Food Facts is a collaborative database, and every contribution is useful for all.
Datuen iturria
Product added on by kiliweb
Last edit of product page on by 5m4u9.
Produktuaren orria -gatik editatua openfoodfacts-contributors, packbot, yuka.sY2b0xO6T85zoF3NwEKvlnB-d9b1gy6VaB3uqGfWmvnfJcy0SMtYxoXlN6g.